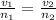Chemistry, 23.03.2020 09:34 liltay12386
At 4.00 L, an expandable vessel contains 0.864 mol of oxygen gas. How many liters of oxygen gas must be added at constant temperature and pressure if you need a total of 1.96 mol of oxygen gas in the vessel?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
At 4.00 L, an expandable vessel contains 0.864 mol of oxygen gas. How many liters of oxygen gas must...
Questions


Social Studies, 07.11.2020 22:50


Biology, 07.11.2020 22:50

Mathematics, 07.11.2020 22:50


English, 07.11.2020 22:50

Mathematics, 07.11.2020 22:50

Physics, 07.11.2020 22:50

Health, 07.11.2020 22:50

Spanish, 07.11.2020 22:50



Mathematics, 07.11.2020 22:50

Computers and Technology, 07.11.2020 22:50

Mathematics, 07.11.2020 22:50


Chemistry, 07.11.2020 22:50

English, 07.11.2020 22:50

History, 07.11.2020 22:50