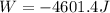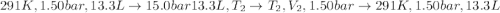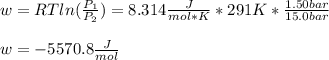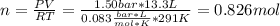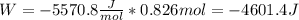An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15.0bar. It then undergoes a reversible isothermal expansion until P=1.50bar. It is then restored to its original state by the extraction of heat at constant pressure. Calculate w for step 2 (P, Vi, T → Pi, V2, T).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
An ideal gas described by Ti=291K, Pi=1.50bar, and Vi=13.3L is heated at constant volume until P=15....
Questions









Business, 27.07.2019 16:40

English, 27.07.2019 16:40





SAT, 27.07.2019 16:40


Geography, 27.07.2019 16:40