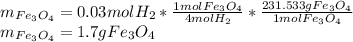Chemistry, 23.03.2020 17:53 eduardavezdemel
Consider the following reaction. 3 Fe(s) + 4 H2O(g) Fe3O4(s) + 4 H2(g) At 900°C, Kc for the reaction is 5.1. If 0.050 mol of H2O(g) and 0.100 mol of Fe(s) are placed in a 1.0 L container at 900°C, how many grams of Fe3O4 are present when equilibrium is established? (This one is somewhat hard.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

You know the right answer?
Consider the following reaction. 3 Fe(s) + 4 H2O(g) Fe3O4(s) + 4 H2(g) At 900°C, Kc for the reaction...
Questions

Biology, 29.07.2019 11:00

Social Studies, 29.07.2019 11:00

Biology, 29.07.2019 11:00

Social Studies, 29.07.2019 11:00

Business, 29.07.2019 11:00


Social Studies, 29.07.2019 11:00


Social Studies, 29.07.2019 11:00



Social Studies, 29.07.2019 11:00

Social Studies, 29.07.2019 11:00


Biology, 29.07.2019 11:00

Biology, 29.07.2019 11:00


Social Studies, 29.07.2019 11:00


Social Studies, 29.07.2019 11:00

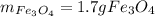
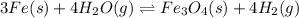
![Kc=\frac{[H_2]^4}{[H_2O]^4}](/tpl/images/0559/0052/b99f2.png)
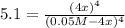
 is obtained as:
is obtained as:![\sqrt[4]{5.1} =\sqrt[4]{[\frac{(4x)}{(0.05M-4x)}]^4}\\\\1.5=\frac{(4x)}{(0.05M-4x)}\\\\x=0.0075M](/tpl/images/0559/0052/4e7d3.png)
![[H_2]_{eq}=4*0.0075\frac{mol}{L}*1.0L=0.03molH_2](/tpl/images/0559/0052/26746.png)