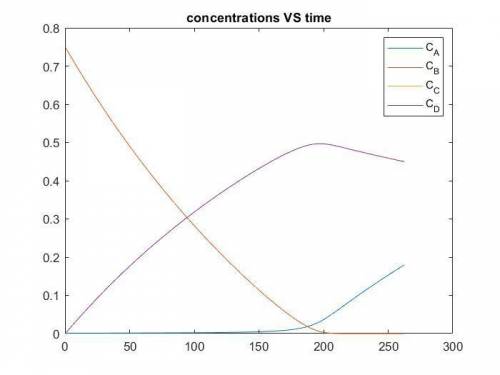
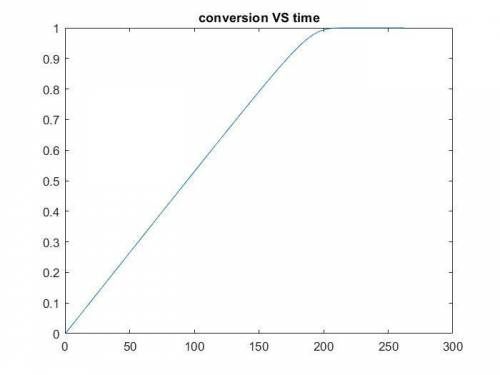
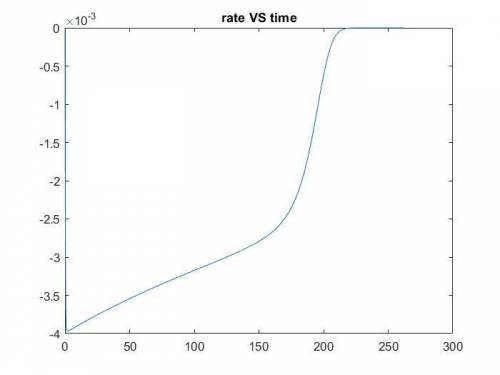
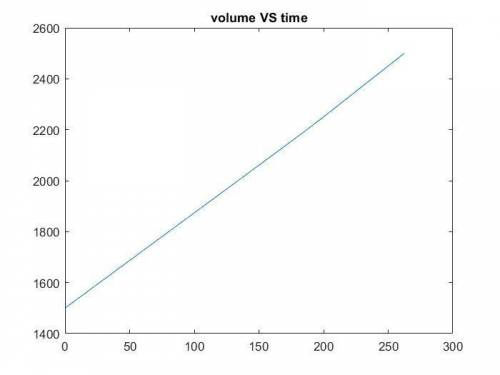
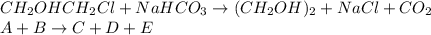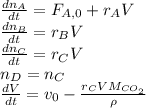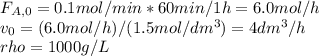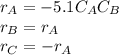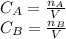The production of ethylene glycol from ethylene chlorohydrin and sodium bicarbonate ↑ is carried out in a semibatch reactor. A 1.5-molar solution of ethylene chlorohydrin is fed at a rate of 0.1 mole/minute to 1500 dm3 of a 0.75-molar solution of sodium bicarbonate. The reaction is elementary and carried out isothermally at 30°C where the specific reaction rate is 5.1 dm3/mol/h. Higher temperatures produce unwanted side reactions. The reactor can hold a maximum of 2500 dm3 of liquid. Assume constant density.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 21.06.2019 18:00
What does earth’s rotation on its axis cause? the tides night and day passing of years phases of the moon
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2
You know the right answer?
The production of ethylene glycol from ethylene chlorohydrin and sodium bicarbonate ↑ is carried out...
Questions


Mathematics, 12.12.2021 20:50

Biology, 12.12.2021 20:50

Mathematics, 12.12.2021 20:50

Physics, 12.12.2021 20:50

Mathematics, 12.12.2021 20:50



Physics, 12.12.2021 20:50




English, 12.12.2021 20:50

Mathematics, 12.12.2021 20:50

World Languages, 12.12.2021 20:50


Mathematics, 12.12.2021 20:50



Mathematics, 12.12.2021 20:50