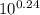Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
A buffer at pH 7.45 is prepared by mixing solutions of KH2PO4 and K2HPO4. Which of the following rat...
Questions

History, 04.02.2020 22:59


Mathematics, 04.02.2020 22:59


Business, 04.02.2020 22:59




Geography, 04.02.2020 22:59

Geography, 04.02.2020 22:59

English, 04.02.2020 22:59

Chemistry, 04.02.2020 23:00


Mathematics, 04.02.2020 23:00




Physics, 04.02.2020 23:00

Computers and Technology, 04.02.2020 23:00

Business, 04.02.2020 23:00

![\frac{[Base]}{[Acid]}](/tpl/images/0559/1224/ebefd.png) = 1.737
= 1.737