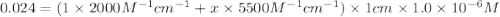The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5500 M-1cm-1. A protein has been isolated that is known to contain one tyrosine residue and an unknown number of tryptophans. A 1.0 micromolar solution of this protein is placed in a 1.0 cm cuvette and the absorbance at 280 nm is measured as 0.024. How many tryptophans are in the protein

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2
You know the right answer?
The molar absorptivity of a tyrosine residue at 280 nm is 2000 M-1cm-1, while for tryptophan it is 5...
Questions









Medicine, 24.10.2019 17:43











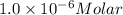


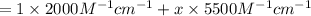
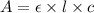 ( Beer-Lambert's law)
( Beer-Lambert's law)