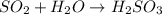Sulfur dioxide from coal-fired power plants combines with water in the atmosphere to produce acid rain. What is the product when one molecule of SO2 reacts with one molecule of watera. two sulfite ions
b. one molecule of sulfuric acid
c. two molecules of sulfurous acid
d. one sulfate ion
e. one molecule of sulfurous acid

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
Sulfur dioxide from coal-fired power plants combines with water in the atmosphere to produce acid ra...
Questions

History, 24.08.2019 12:50

Mathematics, 24.08.2019 12:50




Geography, 24.08.2019 12:50

Biology, 24.08.2019 12:50




History, 24.08.2019 12:50

Biology, 24.08.2019 12:50


Physics, 24.08.2019 12:50


Mathematics, 24.08.2019 12:50

Mathematics, 24.08.2019 12:50

Mathematics, 24.08.2019 12:50


History, 24.08.2019 12:50