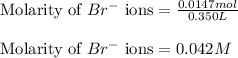Chemistry, 23.03.2020 20:01 lpssprinklezlps
Suppose of iron(II) bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the final molarity of bromide anion in the solution. You can assume the volume of the solution doesn't change when the iron(II) bromide is dissolved in it. Round your answer to significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1
You know the right answer?
Suppose of iron(II) bromide is dissolved in of a aqueous solution of silver nitrate. Calculate the f...
Questions

Mathematics, 08.07.2019 12:00


History, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

History, 08.07.2019 12:00



Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00


Mathematics, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00


Mathematics, 08.07.2019 12:00

Health, 08.07.2019 12:00


Mathematics, 08.07.2019 12:00

History, 08.07.2019 12:00

Mathematics, 08.07.2019 12:00

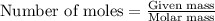
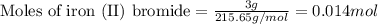
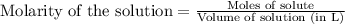 .....(1)
.....(1)
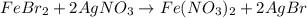
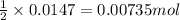 of iron (II) bromide
of iron (II) bromide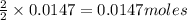 of silver bromide
of silver bromide