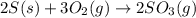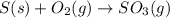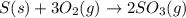Chemistry, 23.03.2020 20:52 jessicagustama
Elemental S reacts with O2 gas to form SO3 gas according to the reaction depicted here. Before a chemical equation can be used to conduct stoichiometric analysis, it must balanced. Type the balanced chemical equation for this reaction between solid sulfur and oxygen gas. Express your answer as a chemical equation including phases.

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
Elemental S reacts with O2 gas to form SO3 gas according to the reaction depicted here. Before a che...
Questions




Mathematics, 27.09.2020 14:01



Chemistry, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01


Mathematics, 27.09.2020 14:01

Physics, 27.09.2020 14:01

Biology, 27.09.2020 14:01





Arts, 27.09.2020 14:01


Mathematics, 27.09.2020 14:01

Geography, 27.09.2020 14:01