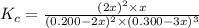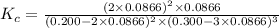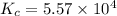In an aqueous solution, iron(III) ions react with iodide ions to give iron(II) ions and triiodide ions, I3-. Suppose the initial concentration of Fe3+ ions is 0.200 M, the initial I- ion concentration is 0.300 M, and the equilibrium concentration of I3- ions is 0.0866 M. What is the value of Kc? 2 Fe3+(aq) + 3 I-(aq) ⇄ 2 Fe2+(aq) + I3-(aq) Kc =

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1
You know the right answer?
In an aqueous solution, iron(III) ions react with iodide ions to give iron(II) ions and triiodide io...
Questions









Computers and Technology, 21.05.2020 03:04

Mathematics, 21.05.2020 03:04

Mathematics, 21.05.2020 03:04




Computers and Technology, 21.05.2020 03:04





Mathematics, 21.05.2020 03:04

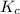 is
is  .
.![[Fe^{3+}]=0.200 M](/tpl/images/0559/4796/129ce.png)
![[I^-]=0.300 M](/tpl/images/0559/4796/84de3.png)
![I_3^{-}=[I_3^{-}]=x=0.0866 M](/tpl/images/0559/4796/0d07b.png)
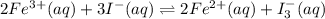
![K_c=\frac{[Fe^{2+}]^2[I_3^{-}]}{[Fe^{3+}]^2[I^-]^3}](/tpl/images/0559/4796/64d23.png)