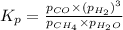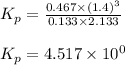Chemistry, 23.03.2020 21:09 darkskinnednune
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 1.5 L flask with 0.60 atm of methane gas and 2.6 atm of water vapor at 47. °C. He then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 1.4 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits x10.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3


Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

You know the right answer?
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions






Mathematics, 23.04.2021 06:30


Mathematics, 23.04.2021 06:30

Chemistry, 23.04.2021 06:30

English, 23.04.2021 06:30

Mathematics, 23.04.2021 06:30

Business, 23.04.2021 06:40


Physics, 23.04.2021 06:40

Physics, 23.04.2021 06:40


Social Studies, 23.04.2021 06:40


Mathematics, 23.04.2021 06:40

Mathematics, 23.04.2021 06:40


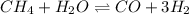
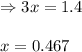
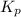 for above equation follows:
for above equation follows: