
Chemistry, 23.03.2020 21:45 angelina12386
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The entropy change when 1.85 moles of liquid Co vaporizes at 3097 °C, 1 atm is J/K.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The...
Questions

Spanish, 05.07.2019 08:00

French, 05.07.2019 08:00


Spanish, 05.07.2019 08:00




Mathematics, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00

English, 05.07.2019 08:00

English, 05.07.2019 08:00

Social Studies, 05.07.2019 08:00

Business, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

Spanish, 05.07.2019 08:00

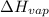 =
= 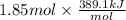
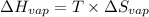
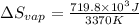
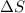 for vaporization is 213.6 J/K.
for vaporization is 213.6 J/K.

