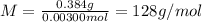A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrated with aqueous NaOH. If 30.0 mL of 0.100 M NaOH is required to reach the equivalence point, what is the molar mass of HA?
(a) 211 g/mol
(b) 128 g/mol
(c) 81.0 g/mol
(d) 37.0 g/mol
(e) 20.3 g/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrat...
Questions



Social Studies, 07.01.2021 17:30


Mathematics, 07.01.2021 17:30

Business, 07.01.2021 17:30

Computers and Technology, 07.01.2021 17:30


Mathematics, 07.01.2021 17:30

Mathematics, 07.01.2021 17:30


Biology, 07.01.2021 17:30


Mathematics, 07.01.2021 17:30







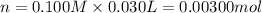
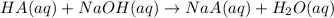
 of HA
of HA