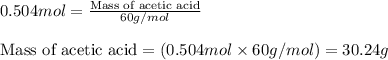Chemistry, 23.03.2020 22:56 j1theking18
In a process for producing acetic acid, oxygen gas is bubbled into acetaldehyde, CH3CHO, containing manganese(II) acetate (catalyst) under pressure at 60°C. 2CH3CHO(l) + O2(g) → 2HC2H3O2(l) In a laboratory test of this reaction, 22.2 g CH3CHO and 12.6 g O2 were put into a reaction vessel. We wish to predict the following: a) How many grams of acetic acid can be produced by this reaction from these amounts of reactants?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
In a process for producing acetic acid, oxygen gas is bubbled into acetaldehyde, CH3CHO, containing...
Questions


Mathematics, 23.02.2021 03:30




Mathematics, 23.02.2021 03:30


Mathematics, 23.02.2021 03:30

Social Studies, 23.02.2021 03:40

Mathematics, 23.02.2021 03:40



Mathematics, 23.02.2021 03:40

Mathematics, 23.02.2021 03:40

Mathematics, 23.02.2021 03:40

History, 23.02.2021 03:40

Mathematics, 23.02.2021 03:40



Mathematics, 23.02.2021 03:40

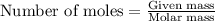 .....(1)
.....(1)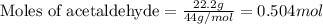
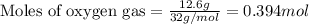

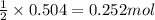 of oxygen gas
of oxygen gas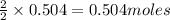 of acetic acid
of acetic acid