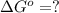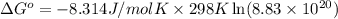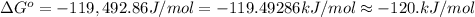In the activity, click on the E∘cell and Keq quantities to observe how they are related. Use this relation to calculate Keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. The two half-reactions that occur in the cell are
Cu2+(aq)+2e−→Cu(s) and Co(s)→Co2+(aq)+2e−
The net reaction is
Cu2+(aq)+Co(s)→Cu(s)+Co2+(aq)
Use the given standard reduction potentials in your calculation as appropriate. ( Keq=5.88*10^20)
In the activity, click on the Keq and ΔG∘ quantities to observe how they are related.
Calculate ΔG∘ using this relationship and the equilibrium constant (Keq) obtained in Part A at T=298K: Keq=5.88*10^20

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
In the activity, click on the E∘cell and Keq quantities to observe how they are related. Use this re...
Questions

History, 27.10.2020 23:30

History, 27.10.2020 23:30


Mathematics, 27.10.2020 23:30


History, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Health, 27.10.2020 23:30

French, 27.10.2020 23:30






Mathematics, 27.10.2020 23:30



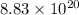 .
.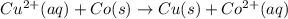
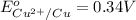
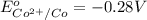
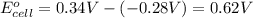
![E^o_{cell}=\frac{0.592}{n}\log[K_c]](/tpl/images/0559/8894/48eca.png)
![0.62 V=\frac{0.0592}{2}\log[K_c]](/tpl/images/0559/8894/f0d8e.png)
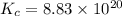
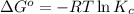
 = standard Gibbs free energy =
= standard Gibbs free energy = 
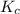 = Equilibrium constant
= Equilibrium constant