Given the following reaction:
Mg(OH)2 + 2HCI -> MgCl2 + 2H2O
How many mol...

Chemistry, 23.03.2020 23:54 onlymyworld27
Given the following reaction:
Mg(OH)2 + 2HCI -> MgCl2 + 2H2O
How many moles of MgCl, will be produced from 32.0 g of Mg(OH)2, assuming HCl is available in excess?
moles (round to three significant figures)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

You know the right answer?
Questions

Mathematics, 15.01.2020 00:31

Mathematics, 15.01.2020 00:31


English, 15.01.2020 00:31



History, 15.01.2020 00:31

Social Studies, 15.01.2020 00:31

Mathematics, 15.01.2020 00:31


Business, 15.01.2020 00:31

English, 15.01.2020 00:31


Geography, 15.01.2020 00:31


Mathematics, 15.01.2020 00:31

Mathematics, 15.01.2020 00:31


History, 15.01.2020 00:31

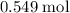 (three significant figures.)
(three significant figures.) 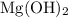 :
: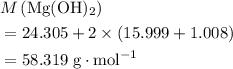 .
.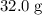 of
of 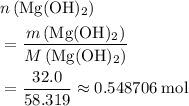 .
.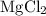 . In other words, for each mole of
. In other words, for each mole of 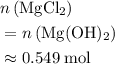 .
.

