
Chemistry, 24.03.2020 00:00 paolaviviana
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C. 2 H2S(g) 2 H2(g) + S2(g) If 0.31 mol H2S is placed in a 4.1 L container, what is the equilibrium concentration of H2(g) at 700°C?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C....
Questions



English, 03.09.2021 06:10


English, 03.09.2021 06:10

Mathematics, 03.09.2021 06:10

Mathematics, 03.09.2021 06:10

Mathematics, 03.09.2021 06:10

Mathematics, 03.09.2021 06:10


English, 03.09.2021 06:10


Mathematics, 03.09.2021 06:10




Mathematics, 03.09.2021 06:10

English, 03.09.2021 06:10

Health, 03.09.2021 06:10

Physics, 03.09.2021 06:10

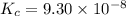 }
}![[concentration]=\frac{moles}{volume (L)}](/tpl/images/0559/9416/7e1bc.png)
![[H_2S]=\frac{0.31 mol}{4.1 L}=0.076 M](/tpl/images/0559/9416/b2f14.png)

![K_c=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0559/9416/3ac5e.png)

![[H_2]=2x=2\times 0.00051 M=0.0010 M](/tpl/images/0559/9416/46b67.png)


