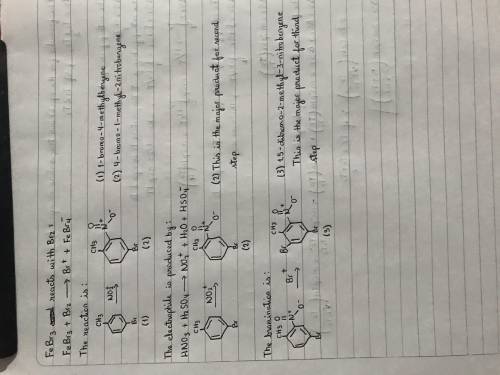
Chemistry, 24.03.2020 01:23 hayden6920
Draw the structure of the product of each step in the following three-step synthesis. Show the formal charges, if applicable. As a start, the benzene ring is drawn for you in each product. Although the first step produces a mixture of isomers, the para isomer is isolated as the sole product of interest and used in the second step. Give only the major product for the second and third steps.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Draw the structure of the product of each step in the following three-step synthesis. Show the forma...
Questions



Mathematics, 25.06.2019 05:20

Mathematics, 25.06.2019 05:20

Business, 25.06.2019 05:20



Mathematics, 25.06.2019 05:20


Mathematics, 25.06.2019 05:20









Computers and Technology, 25.06.2019 05:20

English, 25.06.2019 05:30