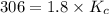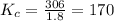Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Carbon monoxide replaces oxygen in oxygenated hemoglobin according to the reaction: HbO2(aq) + CO(aq...
Questions

Business, 01.02.2021 23:10


Mathematics, 01.02.2021 23:10


Mathematics, 01.02.2021 23:10

Spanish, 01.02.2021 23:10



Spanish, 01.02.2021 23:10


Mathematics, 01.02.2021 23:10

Mathematics, 01.02.2021 23:10



Biology, 01.02.2021 23:10


Mathematics, 01.02.2021 23:10


Spanish, 01.02.2021 23:10

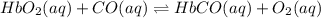
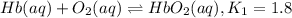
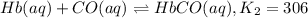
![K_1=\frac{[HbO_2]}{[Hb][O_2]}](/tpl/images/0560/2528/dcc99.png)
![[Hb]=\frac{[HbO_2]}{[K_1][O_2]}](/tpl/images/0560/2528/1a6fb.png) ..[1]
..[1]![K_2=\frac{[HbCO]}{[Hb][CO]}](/tpl/images/0560/2528/abc0a.png) ..[2]
..[2]![K_c=\frac{[HbCO][O_2]}{[HbO_2][CO]}](/tpl/images/0560/2528/bc291.png) ..[3]
..[3]![K_2=\frac{[HbCO]}{\frac{[HbO_2]}{[K_1][O_2]}\times [CO]}](/tpl/images/0560/2528/e05b3.png)
![K_2=K_1\times \frac{[HbCO][O_2]}{[HbO_2][CO]}](/tpl/images/0560/2528/e36c4.png)
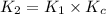 ( using [3])
( using [3])