

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1


You know the right answer?
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrol...
Questions


Mathematics, 23.10.2020 18:50


Arts, 23.10.2020 18:50









Mathematics, 23.10.2020 18:50


English, 23.10.2020 18:50

History, 23.10.2020 18:50

English, 23.10.2020 18:50

Mathematics, 23.10.2020 18:50


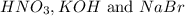
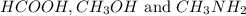
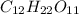
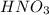
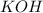
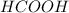
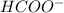 ion and
ion and 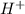 ion. So, it is a weak electrolyte.
ion. So, it is a weak electrolyte.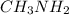
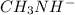 ion and
ion and 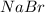
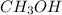
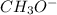 ion and
ion and 

