
Chemistry, 24.03.2020 02:26 johngayden46
Two stones resembling diamonds are suspected of being fakes. To determine if the stones might be real, the mass and volume of each are measured. Both stones have the same volume, 0.15 cm^3. However, stone A has a mass of 0.52 g, and stone B has a mass of 0.42 g.
A) If diamond has a density of 3.5 g/cm^3, could either of the stones be real diamonds? Explain.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Two stones resembling diamonds are suspected of being fakes. To determine if the stones might be rea...
Questions

History, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40

Chemistry, 03.08.2021 18:40











Mathematics, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40





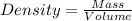
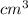 .
.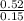 = 3.47
= 3.47 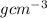 or after rounding off we get 3.5
or after rounding off we get 3.5 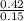 = 2.8
= 2.8 

