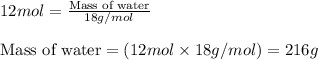Chemistry, 24.03.2020 02:44 musicaljay1276
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g) Which statement is correct about the complete combustion of 3.00 mole of propane, C3H8? \rm C_3H_8(g) + 5 O_2(g) --> 3 CO_2(g) + 4 H_2O(g)Which statement is correct about the complete combustion of 3.00 mole of propane, \rm C_3H_8?1. 12.00 mol H2O are produced.2. 3.00 g CO2 are produced.3. 3.00 mol CO2 are produced.4. 12.00 g H2O are produced

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g...
Questions



Mathematics, 30.12.2019 03:31



Computers and Technology, 30.12.2019 03:31

Mathematics, 30.12.2019 03:31


Geography, 30.12.2019 03:31

Mathematics, 30.12.2019 03:31


Mathematics, 30.12.2019 03:31



Mathematics, 30.12.2019 03:31

Social Studies, 30.12.2019 03:31


Mathematics, 30.12.2019 03:31


English, 30.12.2019 03:31

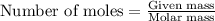 ......(1)
......(1)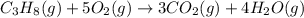
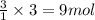 of carbon dioxide
of carbon dioxide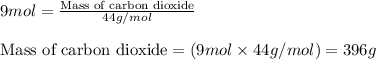
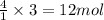 of water
of water