

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

You know the right answer?
The equilibrium constant is equal to 5.00 at 1300 K for the reaction:2 SO2(g) + O2(g) ⇌ 2 SO3(g). If...
Questions





Mathematics, 20.06.2019 18:04


History, 20.06.2019 18:04

World Languages, 20.06.2019 18:04

Mathematics, 20.06.2019 18:04


Mathematics, 20.06.2019 18:04



History, 20.06.2019 18:04






Computers and Technology, 20.06.2019 18:04

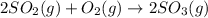
![Q=\frac{[SO_3]^2}{[SO_2]^2[O_2]}](/tpl/images/0560/3663/65406.png)
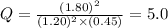
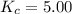
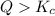 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.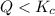 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.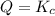 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.


