
Chemistry, 24.03.2020 05:31 CurlyheadShay
Wrting an equilibrium constant for a reaction sequence Hydrogen is manufactured on an industrial scale by this sequence of reactions: CH,( H, O()Co()+3H2() co(g) +H2O(g) CO2(g) + H2(g) K, The net reaction is: CH4(g)+2H2O(g) CO2(g)+4H2(g) Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K and K If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
Wrting an equilibrium constant for a reaction sequence Hydrogen is manufactured on an industrial sca...
Questions




Social Studies, 28.08.2019 18:20




Mathematics, 28.08.2019 18:20

Chemistry, 28.08.2019 18:20


History, 28.08.2019 18:20

Business, 28.08.2019 18:20








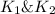 :
: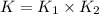
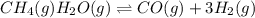
![K_1=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0560/6092/ad23e.png)

![K_2=\frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0560/6092/c2b62.png)

![K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}](/tpl/images/0560/6092/3e51b.png)
![K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}\times \frac{[CO]}{[CO]}](/tpl/images/0560/6092/b92b1.png)
![K=\frac{[CO][H_2]^3}{[CH_4][H_2O]}\times \frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0560/6092/2a778.png)


