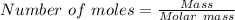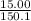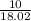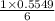Chemistry, 24.03.2020 05:29 lberman2005p77lfi
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limiting reactant is used up. Calculate the mass of H2S (34.08 g/mol) that can be produced from these reactants. Notice that you will need to balance the reaction equation.
___Al2S3(s)+ ___H2O > ___Al(OH)3(s)+ ___H2S(g)
a. 13.89 g
b. 10.21 g
c. 19.67 gd. 9.456 g
e. 1.108 g

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2
You know the right answer?
15.00 g of aluminum sulfide (150.1 g/mol) and 10.00 g of water (18.02 g/mol) react until the limitin...
Questions


Mathematics, 17.01.2021 03:30


History, 17.01.2021 03:30



Social Studies, 17.01.2021 03:30



English, 17.01.2021 03:30

Mathematics, 17.01.2021 03:30

Arts, 17.01.2021 03:30


Mathematics, 17.01.2021 03:30

Mathematics, 17.01.2021 03:30




Arts, 17.01.2021 03:30

Mathematics, 17.01.2021 03:30