
Chemistry, 24.03.2020 07:40 lexybellx3
I point
Given the ionization constant equation:
Kw = [H+][OH-]=1.0 x 10-14
For water at 25°C, which statement is true?
1) [H+] = [OH-]
2) [H+]> [OH-]
3) [H+] = 1.0 10-14 M
4) [OH-] = 1.0 × 10-14 M

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
I point
Given the ionization constant equation:
Kw = [H+][OH-]=1.0 x 10-14
For wat...
Given the ionization constant equation:
Kw = [H+][OH-]=1.0 x 10-14
For wat...
Questions




Mathematics, 28.01.2020 14:08


Mathematics, 28.01.2020 14:08

Mathematics, 28.01.2020 14:08

Mathematics, 28.01.2020 14:08




English, 28.01.2020 14:08

Mathematics, 28.01.2020 14:08

English, 28.01.2020 14:08



Advanced Placement (AP), 28.01.2020 14:08


English, 28.01.2020 14:08

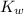 =1.0 ×
=1.0 × 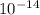 at 25°C
at 25°C behind) molecule ad gets attached to another water molecule leading to the formation of the hydronium ion(
behind) molecule ad gets attached to another water molecule leading to the formation of the hydronium ion(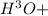 ,hydronium ion).
,hydronium ion).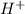 and
and 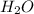 +
+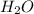 →
→


