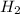Chemistry, 24.03.2020 16:19 juanaaraujo1104
Base your answer on the information below and your knowledge of chemistry. Methanol can be manufactured by a reaction that is reversible. In the reaction, carbon monoxide gas and hydrogen gas react using a catalyst. The equation below represents this system at equilibrium. CO(g) 2H2(g) CH3OH(g) energy. Explain, in terms of collision theory, why increasing the concentration of H2(g) in this system will increase the concentration of CH3OH(g).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement about sound is not true? a. air particles travel with sound waves. b. sound waves cannot travel through a vacuum. c. sound waves exist even if no one hears them. d. air particles vibrate along the path of a sound wave.
Answers: 1

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
Base your answer on the information below and your knowledge of chemistry. Methanol can be manufactu...
Questions



Mathematics, 18.10.2020 08:01

History, 18.10.2020 08:01




English, 18.10.2020 08:01

Social Studies, 18.10.2020 08:01



English, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01




French, 18.10.2020 08:01


Advanced Placement (AP), 18.10.2020 08:01

![Rate=k[CO]^x[H_2]^y](/tpl/images/0560/8875/426a0.png)
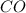 and
and