
Chemistry, 24.03.2020 18:13 doodndns4484
When each of the following equilibria is disturbed by increasing the pressure as a result of decreasing the volume, does the number of moles of reaction products increase, decrease, or remain the same?
1. CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
2. 2CO(g) ⇌ C(s) + CO2(g)
3. N2O4(g) ⇌ 2NO2(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
When each of the following equilibria is disturbed by increasing the pressure as a result of decreas...
Questions

Chemistry, 04.02.2021 18:30


Social Studies, 04.02.2021 18:30


Arts, 04.02.2021 18:30


Mathematics, 04.02.2021 18:30


Mathematics, 04.02.2021 18:30




Social Studies, 04.02.2021 18:30

Mathematics, 04.02.2021 18:30

Mathematics, 04.02.2021 18:30


Biology, 04.02.2021 18:30

Mathematics, 04.02.2021 18:30


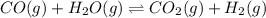 , remain the same.
, remain the same.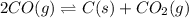 ,increase.
,increase.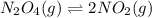 , decrease.
, decrease.




