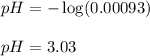Chemistry, 24.03.2020 17:44 hughesbella
Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 7.9 × 10-5, Ka2 = 1.6 × 10-12). Calculate the pH of a solution that contains 2.1 mg acid per mL water. (Assume that only the first ionization is important in determining pH.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 7.9 × 10-5, Ka2 = 1.6 × 10-12). Calculat...
Questions

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

English, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Social Studies, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

History, 13.09.2020 21:01

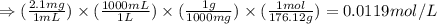
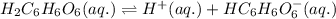
![Ka_1=\frac{[H^+][HC_6H_6O_6^{-}]}{[H_2C_6H_6O_6]}](/tpl/images/0560/9865/81fa9.png)
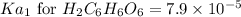
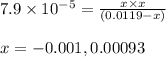
![pH=-\log[H^+]](/tpl/images/0560/9865/cf945.png)
![[H^+]=0.00093M](/tpl/images/0560/9865/52e6b.png)