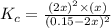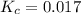When heated, hydrogen sulfide gas decomposes according to the equation: 2H2S(g) 2 H2(g) + S2(g) A 3.75 gram sample of H2S(g) is introduced into an evacuated rigid 0.75 L container. The sealed container is heated to 483 K and 2.42 x 10 –2 mol of S2 gas is present at equilibrium. a. Write the expression for the equilibrium constant, Kc, for the reaction above.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2


Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
When heated, hydrogen sulfide gas decomposes according to the equation: 2H2S(g) 2 H2(g) + S2(g) A...
Questions




History, 16.10.2021 20:40


Mathematics, 16.10.2021 20:40

History, 16.10.2021 20:40

Mathematics, 16.10.2021 20:40

Mathematics, 16.10.2021 20:40

History, 16.10.2021 20:40

Biology, 16.10.2021 20:40

Chemistry, 16.10.2021 20:40

Mathematics, 16.10.2021 20:40


Mathematics, 16.10.2021 20:40


Biology, 16.10.2021 20:40


Mathematics, 16.10.2021 20:40

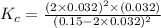
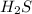 =
= 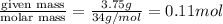
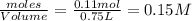
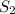 =
= 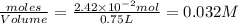
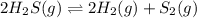
![K_c=\frac{[H_2]^2\times [S_2]}{[H_2S]^2}](/tpl/images/0561/1522/13e21.png)