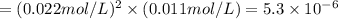Chemistry, 24.03.2020 19:20 ginachuquiano450
At a certain temperature (probably not 25 ºC), the solubility of silver sulfate, Ag₂SO₄, is 0.011 mol/L. Calculate its solubility product constant for this temperature. SIG. FIG. (required because number is small) Solubility product constants are very temperature sensitive. They are generally reported at 25 ºC. Not necessarily using this temperature allows me some flexibility.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
At a certain temperature (probably not 25 ºC), the solubility of silver sulfate, Ag₂SO₄, is 0.011 mo...
Questions


English, 30.03.2021 20:30

Advanced Placement (AP), 30.03.2021 20:30

History, 30.03.2021 20:30

Mathematics, 30.03.2021 20:30

English, 30.03.2021 20:30

History, 30.03.2021 20:30

Computers and Technology, 30.03.2021 20:30




Mathematics, 30.03.2021 20:30



Mathematics, 30.03.2021 20:30




History, 30.03.2021 20:30

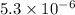 its solubility product constant for this temperature.
its solubility product constant for this temperature. 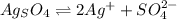
![[Ag_2SO_4]=0.011 mol/L](/tpl/images/0561/1517/1abc0.png)
![[Ag^+]=2\times [Ag_2SO_4]=2\times 0.011 mol/L = 0.022 mol/L](/tpl/images/0561/1517/4e43a.png)
![[SO_4^{2-}]=1\times [Ag_2SO_4]=1\times 0.011 mol/L = 0.011 mol/L](/tpl/images/0561/1517/739e3.png)
![K_{sp}=[Ag^+]^2[SO_4^{2-}]](/tpl/images/0561/1517/a7e5d.png)