
Chemistry, 24.03.2020 19:17 DaylaReevaFEEVA5040
Tin(IV) sulfide, SnS 2 , a yellow pigment, can be produced using the following reaction. SnBr 4 ( aq ) + 2 Na 2 S ( aq ) ⟶ 4 NaBr ( aq ) + SnS 2 ( s ) Suppose a student adds 47.7 mL of a 0.474 M solution of SnBr 4 to 43.4 mL of a 0.179 M solution of Na 2 S . Identify the limiting reactant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3


Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Tin(IV) sulfide, SnS 2 , a yellow pigment, can be produced using the following reaction. SnBr 4 ( aq...
Questions

Mathematics, 02.03.2021 07:20

Mathematics, 02.03.2021 07:20

Mathematics, 02.03.2021 07:20




Health, 02.03.2021 07:20


Chemistry, 02.03.2021 07:20

Mathematics, 02.03.2021 07:20


Biology, 02.03.2021 07:20

Social Studies, 02.03.2021 07:20

Mathematics, 02.03.2021 07:20

Mathematics, 02.03.2021 07:20


Physics, 02.03.2021 07:20

Social Studies, 02.03.2021 07:20

Social Studies, 02.03.2021 07:20

English, 02.03.2021 07:20

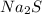 is the limiting reagent
is the limiting reagent 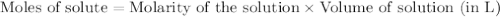 .....(1)
.....(1)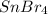 solution = 0.474 M
solution = 0.474 M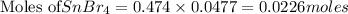

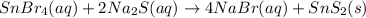
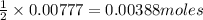 of
of 

