Chemistry, 24.03.2020 19:14 roseemariehunter12
Initially a NaOH solution was standardized by titration with a sample of potassium hydrogenphthalate, KHC8H4O4, a monoprotic acid often used as a primary standard. A sample of pureKHC8H4O4 weighing 1.518 grams was dissolved in water and titrated with the NaOH solution. Toreach the equivalence point, 26.90 milliliters of base was required. Calculate the molarity of theNaOH solution. (Molecular weight: KHC8H4O4 = 204.2)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Initially a NaOH solution was standardized by titration with a sample of potassium hydrogenphthalate...
Questions

Social Studies, 06.11.2020 22:30


Chemistry, 06.11.2020 22:30



Social Studies, 06.11.2020 22:30


Social Studies, 06.11.2020 22:30

Chemistry, 06.11.2020 22:30


English, 06.11.2020 22:30



Mathematics, 06.11.2020 22:30


Health, 06.11.2020 22:30

Social Studies, 06.11.2020 22:30



Biology, 06.11.2020 22:30

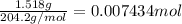
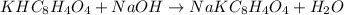
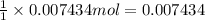 of NaOH
of NaOH