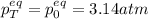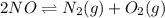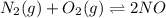Chemistry, 24.03.2020 19:22 brisamauro27
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the total pressure of the mixture at equilibrium is 3.14 atm . N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) K p = 0.101 at 2000 ° C In which direction does the reaction proceed after heating to 2000 °C? The reaction is at equilibrium. The reaction proceeds toward the reactants. The reaction proceeds toward the products.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the...
Questions

Computers and Technology, 30.07.2019 15:40

Health, 30.07.2019 15:40



History, 30.07.2019 15:40







History, 30.07.2019 15:40



History, 30.07.2019 15:40





Biology, 30.07.2019 15:40