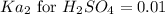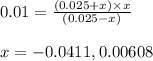Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
Suppose a 0.025M aqueous solution of sulfuric acid (H2SO4) is prepared. Calculate the equilibrium mo...
Questions


Mathematics, 10.08.2021 22:00




Computers and Technology, 10.08.2021 22:00

Computers and Technology, 10.08.2021 22:00


Business, 10.08.2021 22:00


Computers and Technology, 10.08.2021 22:00




Physics, 10.08.2021 22:10

Mathematics, 10.08.2021 22:10


Mathematics, 10.08.2021 22:10


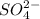 at equilibrium is 0.00608 M
at equilibrium is 0.00608 M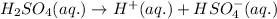
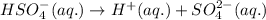
![Ka_2=\frac{[H^+][SO_4^{2-}]}{[HSO_4^-]}](/tpl/images/0561/2306/83003.png)