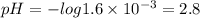Consider a 1.6 × 10-3 M solution of HNO3. Which of the following statements is NOT true? Consider a 1.6 × 10-3 M solution of HNO3. Which of the following statements is NOT true? This solution could dissolve metal. This solution could neutralize a base. This solution would turn litmus to red. This solution has a pH of 11.20. none of the above

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

You know the right answer?
Consider a 1.6 × 10-3 M solution of HNO3. Which of the following statements is NOT true? Consider a...
Questions




Mathematics, 01.04.2021 08:40

Mathematics, 01.04.2021 08:40

English, 01.04.2021 08:40

History, 01.04.2021 08:40



Mathematics, 01.04.2021 08:40

Mathematics, 01.04.2021 08:40



Physics, 01.04.2021 08:40


Mathematics, 01.04.2021 08:40

English, 01.04.2021 08:40


History, 01.04.2021 08:40