
Chemistry, 24.03.2020 20:14 nehakarakkattu
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an enthalpy of 498 kJ/mol , and the standard enthalpy of formation of ClO2 102.5 kJ/mol , calculate the value for the enthalpy of formation per mole of ClO(g). What is the value for the enthalpy of formation per mole of ClO(g)? Enter your answer numerically, in terms of kJ, and to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 18:00
Temperature and kinetic energy are proportional. a) adirectly b) directly c) indirectly
Answers: 2

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

You know the right answer?
Given that a chlorine-oxygen bond has an enthalpy of 243 kJ/mol , an oxygen-oxygen bond has an entha...
Questions

Mathematics, 19.05.2021 02:00

Mathematics, 19.05.2021 02:00


Spanish, 19.05.2021 02:00



Mathematics, 19.05.2021 02:00


Social Studies, 19.05.2021 02:00

Mathematics, 19.05.2021 02:00

Mathematics, 19.05.2021 02:00




Mathematics, 19.05.2021 02:10


Chemistry, 19.05.2021 02:10


Mathematics, 19.05.2021 02:10

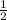
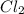 (g) +
(g) + 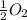 (g) → ClO(g)
(g) → ClO(g) 


