Chemistry, 24.03.2020 21:41 kaffolter25
How many grams of sodium acetate ( molar mass = 83.06 g/mol ) must be added to 1.00 Liter of a 0.200 M acetic acid solution to make a buffer with a pH of 5.00. Assume no volume change with addition of the solid. ( Ka of acetic acid = 1.8 x 10 -5 )

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1


Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

You know the right answer?
How many grams of sodium acetate ( molar mass = 83.06 g/mol ) must be added to 1.00 Liter of a 0.200...
Questions



History, 08.04.2020 21:12

Biology, 08.04.2020 21:12

History, 08.04.2020 21:12

Mathematics, 08.04.2020 21:12

Biology, 08.04.2020 21:12

Biology, 08.04.2020 21:12




Mathematics, 08.04.2020 21:12

Biology, 08.04.2020 21:12

Social Studies, 08.04.2020 21:12

History, 08.04.2020 21:12




Mathematics, 08.04.2020 21:12

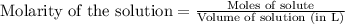

![pH=pK_a+\log(\frac{[\text{salt}]}{[\text{acid}]})](/tpl/images/0561/6049/4d4ed.png)
![pH=pK_a+\log(\frac{[CH_3COONa]}{[CH_3COOH]})](/tpl/images/0561/6049/05ea7.png)
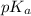 = negative logarithm of acid dissociation constant of acetic acid = 4.74
= negative logarithm of acid dissociation constant of acetic acid = 4.74![[CH_3COONa]=?mol](/tpl/images/0561/6049/bb071.png)
![[CH_3COOH]=0.200mol](/tpl/images/0561/6049/944f0.png)
![5=4.74+\log(\frac{[CH_3COONa]}{0.200})](/tpl/images/0561/6049/c8eb7.png)
![[CH_3COONa]=0.364mol](/tpl/images/0561/6049/4c5e2.png)