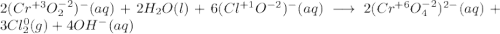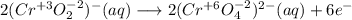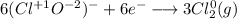Chemistry, 24.03.2020 20:58 grayjasmine46
Consider the following balanced redox reaction: 2CrO2-(aq) + 2H2O(l) + 6ClO-(aq) LaTeX: \longrightarrow⟶ 2CrO42-(aq) + 3Cl2(g) + 4OH-(aq) 1. Which species is being oxidized? 2. Which species is being reduced? 3. Which species is the oxidizing agent? 4. Which species is the reducing agent? 5. How many electrons are being transferred? Hint: If you were to balance this equation how many electrons would be in each half-reaction? That is how many electrons are transferred.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Consider the following balanced redox reaction: 2CrO2-(aq) + 2H2O(l) + 6ClO-(aq) LaTeX: \longrightar...
Questions


English, 24.09.2019 06:30


Biology, 24.09.2019 06:30

Social Studies, 24.09.2019 06:30

English, 24.09.2019 06:30

Biology, 24.09.2019 06:30



Chemistry, 24.09.2019 06:30


Mathematics, 24.09.2019 06:30


History, 24.09.2019 06:30