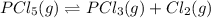Chemistry, 24.03.2020 21:15 elizzabel1944
Consider the system at equilibrium. PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) How will increasing the concentration of PCl5 shift the equilibrium? to the left to the right no effect How will increasing the concentration of PCl3 shift the equilibrium? to the right no effect to the left How will increasing the pressure by adding argon gas to the reaction mixture, while maintaining a constant volume, shift the equilibrium? to the right to the left no effect

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3


Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
Consider the system at equilibrium. PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) How will increasing...
Questions


Mathematics, 01.03.2021 21:20

Physics, 01.03.2021 21:20

Biology, 01.03.2021 21:20


Mathematics, 01.03.2021 21:20


World Languages, 01.03.2021 21:20

Social Studies, 01.03.2021 21:20



History, 01.03.2021 21:20

Mathematics, 01.03.2021 21:20

Mathematics, 01.03.2021 21:20

History, 01.03.2021 21:20



Mathematics, 01.03.2021 21:20


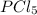 shift the equilibrium to the right side.
shift the equilibrium to the right side.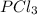 shift the equilibrium to the left.
shift the equilibrium to the left.