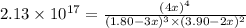Chemistry, 24.03.2020 21:33 BatmanVS1944
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, Kc. 3 A ( g ) + 2 B ( g ) − ⇀ ↽ − 4 C ( g ) K c = 2.13 × 10 17 If, at this temperature, 1.80 mol of A and 3.90 mol of B are placed in a 1.00 L container, what are the concentrations of A, B, and C at equilibrium?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1
You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions




Spanish, 20.10.2020 01:01



History, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01



Mathematics, 20.10.2020 01:01


Biology, 20.10.2020 01:01




Biology, 20.10.2020 01:01

Chemistry, 20.10.2020 01:01

Chemistry, 20.10.2020 01:01

![[A]=\frac{1.80 mol}{1.00 L}=1.80 M](/tpl/images/0561/5634/418e6.png)
![[B]=\frac{3.90 mol}{1.00 L}=3.90 M](/tpl/images/0561/5634/3de81.png)
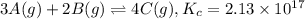
![K_c=\frac{[C]^4}{[A]^3[B]^2}](/tpl/images/0561/5634/6b95d.png)