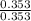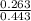A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH is 1.80×10-5 , what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.090 mol HI is added to 1.00 L of the buffer solution. Use H3O+ instead of H+ .

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
A buffer solution is made that is 0.353 M in CH3COOH and 0.353 M in CH3COO- . (1) If Ka for CH3COOH...
Questions




History, 09.12.2021 03:10









Physics, 09.12.2021 03:10






SAT, 09.12.2021 03:10

SAT, 09.12.2021 03:10

![\frac{[Salt]}{[Acid]}](/tpl/images/0561/5667/6743a.png)