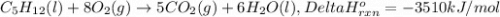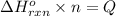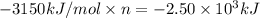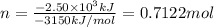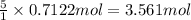Chemistry, 24.03.2020 21:43 morgan3346
The combustion of pentane produces heat according to the following thermochemical equation. C5H12(l) + 8O2(g) → 5CO2(g) + 6H2O(l) ΔH°rxn= –3510 kJ/mol How many grams of CO2 is produced per 2.50 × 103 kJ of heat released?\

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
The combustion of pentane produces heat according to the following thermochemical equation. C5H12(l)...
Questions



Biology, 03.02.2021 19:40

Mathematics, 03.02.2021 19:40



Chemistry, 03.02.2021 19:40


Mathematics, 03.02.2021 19:40






English, 03.02.2021 19:40


Mathematics, 03.02.2021 19:40


Mathematics, 03.02.2021 19:40

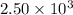 kJ of heat released.
kJ of heat released.