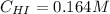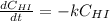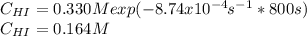Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys t...

Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys this rate law.
Rate= 8.74 x 10^-4 s^1
Suppose a vessel contains HI at a concentration of 0.330M. Calculate the concentration of HI in the vessel 800 seconds later. You may assume no other reaction is important. Round your answer to significant digit

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Questions

Mathematics, 20.09.2020 08:01

English, 20.09.2020 08:01

English, 20.09.2020 08:01

History, 20.09.2020 08:01

English, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01





Mathematics, 20.09.2020 08:01

Chemistry, 20.09.2020 08:01

Social Studies, 20.09.2020 08:01




Computers and Technology, 20.09.2020 08:01

Health, 20.09.2020 08:01