
You're provided with a bottle labled [CoCl2.6H2O] = 0.054 M in 4.60 M HCl. You heat a small volume of the solution in a hot water bath to 50 ∘C. If you determine that [CoCl42-] = 0.034 M at 50 ∘C at equilibrium, what must be the equilibrium concentration of [Cl-] at 50 ∘C?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
You're provided with a bottle labled [CoCl2.6H2O] = 0.054 M in 4.60 M HCl. You heat a small volume o...
Questions




Mathematics, 09.09.2021 16:50

Biology, 09.09.2021 16:50


Biology, 09.09.2021 16:50

English, 09.09.2021 16:50


Biology, 09.09.2021 16:50

English, 09.09.2021 16:50

Mathematics, 09.09.2021 16:50

Mathematics, 09.09.2021 16:50

Mathematics, 09.09.2021 16:50

History, 09.09.2021 16:50




History, 09.09.2021 16:50

![[CoCl_2.6H_2O]](/tpl/images/0561/6622/227f6.png) = 0.054 M
= 0.054 M![[CoCl_4]^{2-}](/tpl/images/0561/6622/84955.png) = 0.034 M
= 0.034 M![[CoCl_2.6H_2O]+2HCl\rightarrow H_2[CoCl_4]+6H_2O](/tpl/images/0561/6622/defc4.png)
![[CoCl_2.6H_2O]+2Cl^-\rightarrow [CoCl_4]^{2-}+6H_2O](/tpl/images/0561/6622/500e9.png)
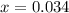
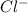 = (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M
= (4.60 - 2x) = [4.60 - 2(0.034)] = 4.532 M

