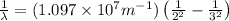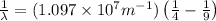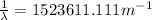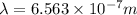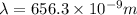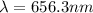Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2

You know the right answer?
A wavelength of 656.3 nm is emitted by the hydrogen atoms in a high-voltage discharge tube. What are...
Questions







Computers and Technology, 11.12.2019 23:31


Computers and Technology, 11.12.2019 23:31











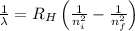
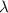 = Wavelength
= Wavelength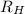 = Rydberg's Constant =
= Rydberg's Constant = 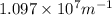
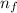 = Higher energy level = 3
= Higher energy level = 3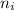 = Lower energy level = 2
= Lower energy level = 2