Chemistry, 24.03.2020 22:28 mckleinrivero
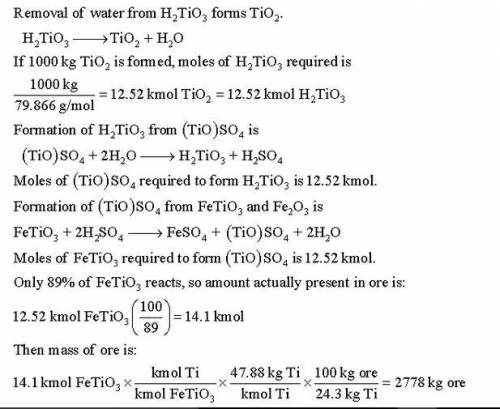
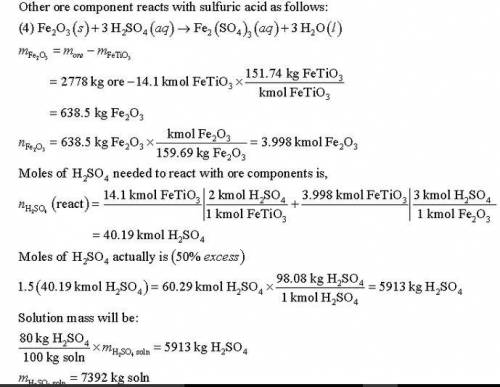
Titanium dioxide (TiO ) is used extensively as a white pigment. It is produced from an ore that contains ilmenite (FeTiO ) and ferric oxide (Fe O ). The ore is digested with an aqueous sulfuric acid solution to produce an aqueous solution of titanyl sulfate [(TiO)SO ] and ferrous sulfate (FeSO ). Water is added to hydrolyze the titanyl sulfate to H TiO , which precipitates, and H SO . The precipitate is then roasted, driving off water and leaving a residue of pure titanium dioxide. (Several steps to remove iron from the intermediate solutions as iron sulfate have been omitted from this description.) Suppose an ore containing 24.3% Ti by mass is digested with an 80% H SO solution, supplied in 50% excess of the amount needed to convert all the ilmenite to titanyl sulfate and all the ferric oxide to ferric sulfate [Fe (SO ) ]. Further suppose that 89% of the ilmenite actually decomposes. Calculate the masses (kg) of ore and 80% sulfuric acid solution that must be fed to produce 1000 kg of pure TiO .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1
You know the right answer?
Titanium dioxide (TiO ) is used extensively as a white pigment. It is produced from an ore that cont...
Questions

English, 02.12.2020 14:40




Social Studies, 02.12.2020 14:40


Chemistry, 02.12.2020 14:40



Mathematics, 02.12.2020 14:40


Social Studies, 02.12.2020 14:50

Mathematics, 02.12.2020 14:50

Biology, 02.12.2020 14:50

Mathematics, 02.12.2020 14:50

Mathematics, 02.12.2020 14:50




Chemistry, 02.12.2020 14:50