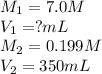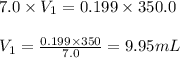Chemistry, 24.03.2020 22:17 sahaitong1844
A chemist must prepare 300.0mL of nitric acid solution with a pH of 0.70 at 25°C. He will do this in three steps: Fill a 300.0mL volumetric flask about halfway with distilled water. Measure out a small volume of concentrated (7.0M) stock nitric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated nitric acid

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
A chemist must prepare 300.0mL of nitric acid solution with a pH of 0.70 at 25°C. He will do this in...
Questions


Spanish, 25.11.2021 18:40

World Languages, 25.11.2021 18:40

Mathematics, 25.11.2021 18:40


Biology, 25.11.2021 18:40

Mathematics, 25.11.2021 18:40

History, 25.11.2021 18:40


Biology, 25.11.2021 18:40

Physics, 25.11.2021 18:40

Social Studies, 25.11.2021 18:40


Business, 25.11.2021 18:40


Social Studies, 25.11.2021 18:40




![pH=-\log[H^+]](/tpl/images/0561/6942/cf945.png)
![0.70=-\log[H^+]](/tpl/images/0561/6942/ee64e.png)
![[H^+]=10^{-0.70}=0.199M](/tpl/images/0561/6942/c6916.png)
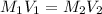
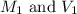 are the molarity and volume of the concentrated nitric acid solution
are the molarity and volume of the concentrated nitric acid solution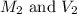 are the molarity and volume of diluted nitric acid solution
are the molarity and volume of diluted nitric acid solution