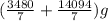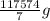Chemistry, 24.03.2020 22:30 2023greenlanden
10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce a 10 mol % aqueous NaOH solution. The NaOH and water are initially at 25 OC. Assume the heat capacity of solution is 2.8 J/g C. How much of heat to be removed to keep the final solution at 10OC?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
10 mol/h of 50 mol % NaOH is mixed with enough 6.5 mol % NaOH to produce a 10 mol % aqueous NaOH sol...
Questions

Computers and Technology, 19.10.2019 19:30

Physics, 19.10.2019 19:30

English, 19.10.2019 19:30

Mathematics, 19.10.2019 19:30

Mathematics, 19.10.2019 19:30

Mathematics, 19.10.2019 19:30





Social Studies, 19.10.2019 19:30

Biology, 19.10.2019 19:30





Mathematics, 19.10.2019 19:30

Mathematics, 19.10.2019 19:30

Social Studies, 19.10.2019 19:30

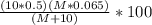
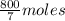
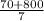
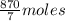
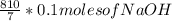
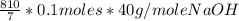
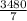 g of NaOH
g of NaOH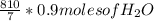
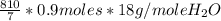
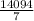 g of H₂O
g of H₂O