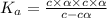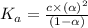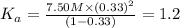Chemistry, 24.03.2020 22:32 allisonlillian
Nitric acid (HNO3) is a strong acid that is completely ionized in aqueous solutions of concentrations ranging from 1% to 10% (1.50 M ). However, in more concentrated solutions, part of the nitric acid is present as un-ionized molecules of HNO3. For example, in a 50% solution (7.50 M ) at 25°C, only 33% of the molecules of HNO3 dissociate into H+ and NO3–. What is the value of Ka for HNO3?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Nitric acid (HNO3) is a strong acid that is completely ionized in aqueous solutions of concentration...
Questions

Mathematics, 23.09.2019 15:10

Computers and Technology, 23.09.2019 15:10


Physics, 23.09.2019 15:10



Spanish, 23.09.2019 15:10


Mathematics, 23.09.2019 15:10

Mathematics, 23.09.2019 15:10

Geography, 23.09.2019 15:10


Biology, 23.09.2019 15:10



Mathematics, 23.09.2019 15:10



History, 23.09.2019 15:10

Social Studies, 23.09.2019 15:10

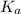 of the nitric acid is 1.2.
of the nitric acid is 1.2.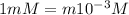
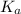

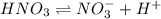
![K_a=\frac{[NO_3^{-}][H^+]}{[HNO_3]}](/tpl/images/0561/7216/4e976.png)